Recent studies have shown that amyloid protein diseases such as Alzheimer’s, Parkinson’s, etc. are very common among the world’s population.
These diseases belong to the class of age-related diseases. Many other diseases and their complications, such as cataracts, diabetes, etc., are associated with the aggregation of proteins and the formation of amyloid. It is expected that in the near future, amyloid diseases will become a serious medical and economic problem in the world.
Joint research conducted within the framework of a joint program under the cooperation agreement between the National Academy of Sciences of Azerbaijan (ANAS) and the National Research Council of Italy (CNR) has opened up new prospects in the fight against amyloid diseases.
On the Azerbaijani side, the research is led by the director of the Institute of Biophysics of ANAS, corresponding member Oktay Gasimov, and on the Italian side – a scientist of the Institute of Chemical Sciences and Technologies (Milan) Laura Ragona. For a long time, the Ph.D. student of the Institute of Biophysics Aida Mammadzade took an active part in the research work.
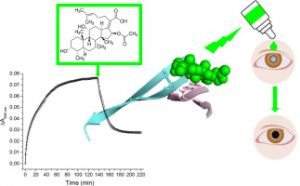
Silk fibroin (SF) is a non-pathological amyloidgenic protein prone, in solution, to the formation of amyloid-like aggregated species, displaying similarities in fibrillation kinetics with pathological amyloids, as widely reported in the literature. Experts show here, on the basis of different biophysical approaches (turbidity, Congo Red assays, CD, DLS and fluorescence), that fusidic acid (FA), a well-known antibiotic, acts on SF as an anti-aggregating agent in a dose-dependent manner, being also able to revert SF aggregation. FA binds to SF inducing changes in the environment of SF aromatic residues. We further provide the proof of principle that FA, already approved as drug on humans and used in ophthalmic preparations, displays its anti-aggregation properties also on lens material derived from cataract surgery and is capable of reducing aggregation. Thus it is suggested that FA can be foreseen as a therapeutic treatment for cataract and other protein aggregation disorders.
Fusidic acid, a known antibiotic, interferes with silk fibroin self-aggregation and is also effective in disaggregating human cataract lens material. Fusidic acid shows a therapeutic potential in cataract prevention. Silk fibroin, an easily available amyloidogenic protein, could represent a useful and inexpensive platform to select aggregation-inhibitors of pathological amyloids.
The important results obtained within the framework of the project were reflected in an article published in the International Journal “Biophysical Chemistry” with a high impact factor.